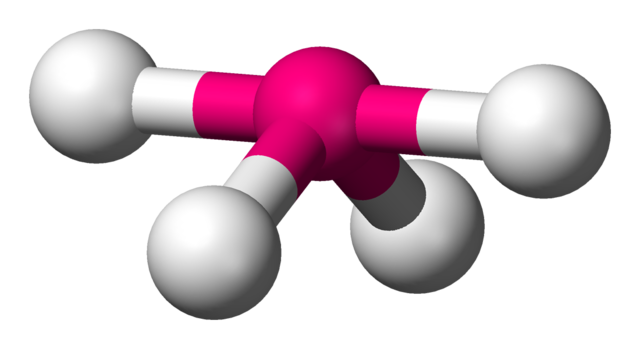
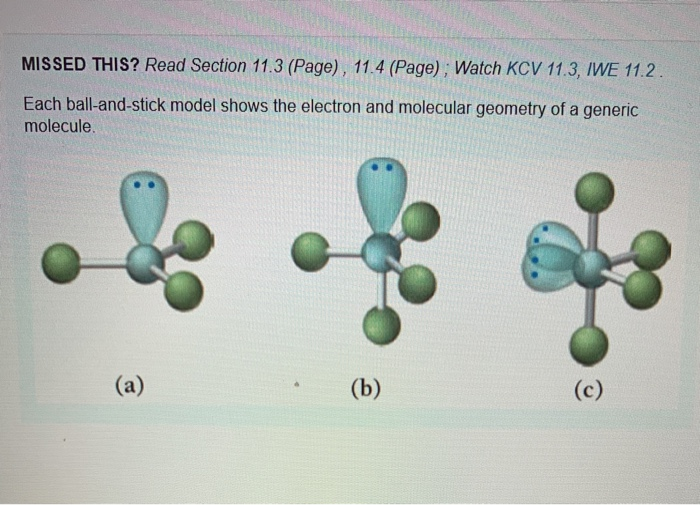
1 SHAPES OF MOLECULES: VSEPR MODEL 1.1 INTRODUCTION • The shapes of molecules tend to be controlled by the number of electrons

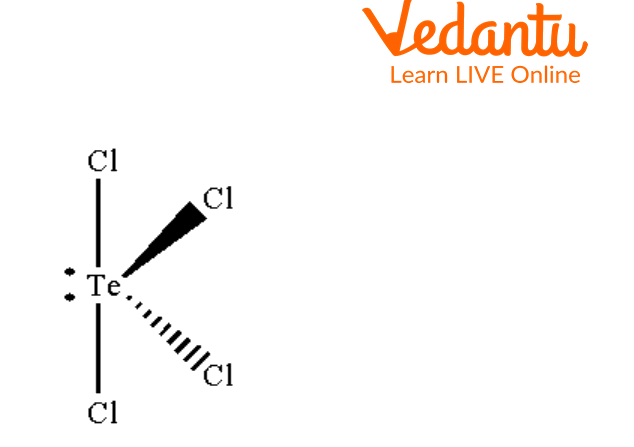
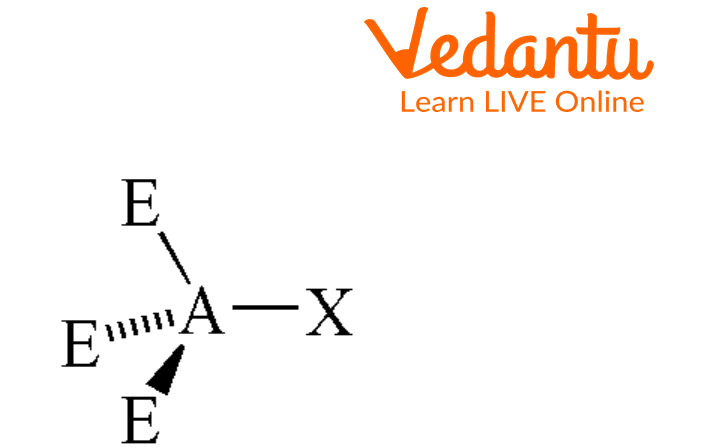
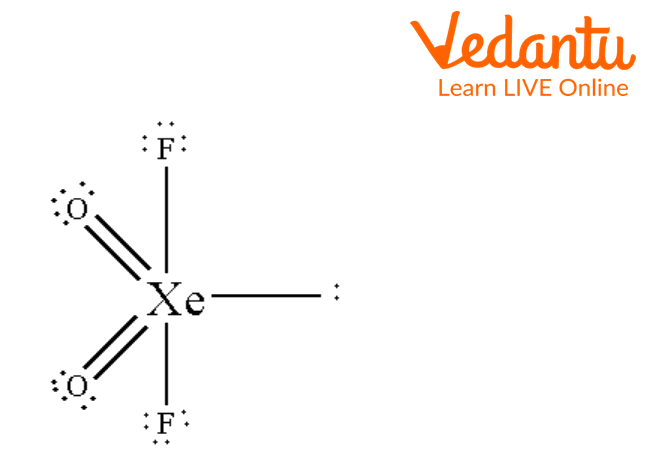
Seesaw Molecular Geometry - Seesaw Shaped Molecules, Lone pairs, Examples & Hybridisation of Seesaw Molecular Geometry
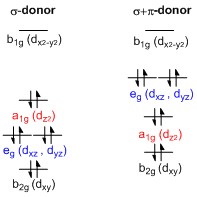
Teaching Three-Dimensional Structural Chemistry Using Crystal Structure Databases. 1. An Interactive Web-Accessible Teaching Sub

What makes a seesaw-shaped molecule instead of a square planar or tetrahedral? I understand that tetrahedron has 0 lone pairs, but what about the others? - Quora
What makes a seesaw-shaped molecule instead of a square planar or tetrahedral? I understand that tetrahedron has 0 lone pairs, but what about the others? - Quora
What makes a seesaw-shaped molecule instead of a square planar or tetrahedral? I understand that tetrahedron has 0 lone pairs, but what about the others? - Quora
1 SHAPES OF MOLECULES: VSEPR MODEL 1.1 INTRODUCTION • The shapes of molecules tend to be controlled by the number of electrons
What makes a seesaw-shaped molecule instead of a square planar or tetrahedral? I understand that tetrahedron has 0 lone pairs, but what about the others? - Quora
Teaching Three-Dimensional Structural Chemistry Using Crystal Structure Databases. 1. An Interactive Web-Accessible Teaching Sub
What makes a seesaw-shaped molecule instead of a square planar or tetrahedral? I understand that tetrahedron has 0 lone pairs, but what about the others? - Quora